Overall Solution
For different scenarios, the establishment of molecular diagnostic service system can effectively meet the needs of epidemic prevention and control,animal disease detection, pet health management detection and parasitic disease detection, etc., which has a wide market space and great social significance.
Overall Solution for SARS-CoV-2
Intended use:
The kit is used for in vitro qualitative detection of the ORF1AB gene of the novel coronavirus (2019-NCOV) in throat swab and sputum samples collected from suspected cases of COVID-19, suspected patients with clustered cases, and other individuals who need to make a diagnosis or differential diagnosis of novel coronavirus infections.
Packing Specifications:
24 Tests/Kit, 48 Tests/Kit, 96 Tests/Kit
Product components:
2019-nCoV reaction unit, buffer IV, magnesium acetate, 2019-nCoV positive control, negative control (G)
SARS-CoV-2 Nucleic Acid Detection Kit(Fluorescent RT-RAA)

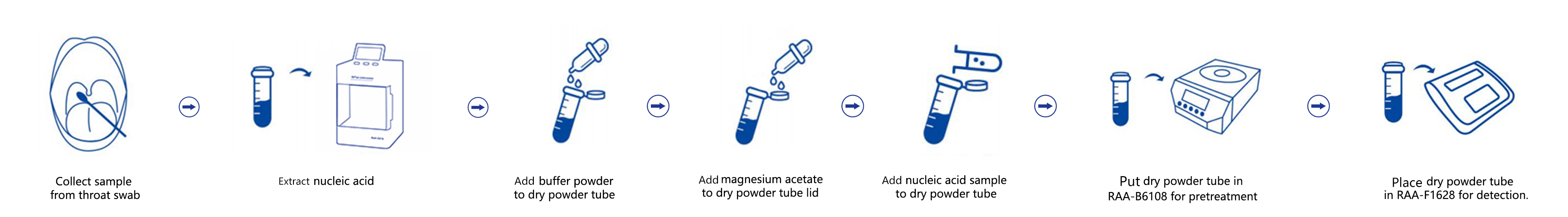
Operation Process

Product Features
Lower Detection Limit: 384 copies/ml
Specificity: no cross-reaction, can detect a variety of mutants
Detection Time: 8-15 min

Storage Conditions And Expiration Date


WeChat Official Account


Service Hotline:
0510-85385531Telephone:
18921157475Address:
4-5F, Building B, Xingye Building, No. 97 Linghu Avenue, Xinwu District, Wuxi CityF
